LUNG TRANSPLANTATION
Dr Brendan Madden MD, MSc, FRCPI, FRCP
Consultant Cardiothoracic Physician and Reader in
Cardiothoracic Medicine St George’s Hospital, London
LEARNING OBJECTIVES
The aim of this section is to provide an overview of lung transplantation
to familiarise you with the type of patients who may be considered for
lung transplantation, the operative techniques available and the postoperative
complications and results.
INTRODUCTION
Lung transplantation has an established role in the management of a variety
of pulmonary vascular and parenchymal lung diseases leading to end stage
respiratory failure. The transplant operations available include single
lung transplantation (SLT), bilateral sequential lung transplantation
(BSLT), heart and lung transplantation (HLT) and lobar transplantation.
INDICATIONS AND CONTRAINDICATIONS TO LUNG TRANSPLANTATION
The main indications for lung transplantation are:
• Severe respiratory failure despite maximal medical therapy.
• Severely impaired quality of life.
• Patient positively wants a transplant.
Only patients who have deteriorating chronic respiratory failure should
be accepted onto the transplant waiting list and in practice the FEV1
is usually less than 30% of the predicted value. Careful psychological
assessment is necessary to exclude patients with intractable psychosocial
instability that may interfere with their ability to cope with the operation
and to comply with the strict postoperative follow up and immunosuppressive
regimes. In most centres the upper age limit is 60 years for SLT and 50
years for HLT and BSLT. Contraindications to lung transplantation are
listed in Table 1. Patients with cushingoid features are excluded until
these changes subside with reduction in steroid therapy. There is concern
that long term steroid therapy in excess of Prednisolone 10mg daily may
adversely affect tissue healing and in particular healing of the major
airway anastomoses after transplantation. A number of risk factors significantly
increase early mortality after lung transplantation. Previous pleurodesis
or thoracic surgery increases the risk of bleeding and attendant complications,
patients on preoperative ventilatory support may have colonisation of
their sputum with resistant bacteria and hence may develop postoperative
infection and combined BSLT or HLT and liver transplantation in cystic
fibrosis patients with respiratory failure and portal hypertension is
associated with increased early mortality.
Question 1.
The following are strong contraindications to lung transplantation:
|
|
|
|
| 1. Non compliance with treatment | ||
| 2. Malignant disease successfully treated 7 years prior to assessment | ||
| 3. Methicillin-resistant staphylococcus aureus in sputum | ||
| 4. Previous thoracic surgery | ||
| 5. Active aspergillus or mycobacterial infection |
SURGICAL PROCEDURES
Heart lung transplantation: With a reduction in the number of donor
organs available worldwide the indications for HLT have been redefined.
In practice HLT is reserved for patients with Eisenmengers syndrome who
have a surgically incorrectable cardiac defect. It has been applied to
patients with pulmonary vascular and parenchymal lung diseases in the
past. This transplant operation is performed via a median sternotomy incision
with cardiopulmonary bypass and the anastomoses are fashioned at the level
of the right atrium, aorta and trachea. The trachea receives an important
blood supply from the coronary to bronchial collateral circulation and
this is not disturbed with HLT. Therefore airway ischaemic complications,
(the major cause of mortality in the early days of lung transplantation)
are uncommon.
Another cited advantage of HLT is domino cardiac transplantation. In this
procedure the heart from a patient who receives HLT for conditions other
than Eisenmengers syndrome can, if healthy, be successfully transplanted
into a patient requiring cardiac transplantation alone, so long as there
is no severe irreversible elevation in pulmonary vascular resistance.
The results of this procedure are very encouraging and in addition it
offers advantages of short organ ischaemic time and non exposure of the
donor heart to the effects of brain stem death.
Bilateral lung transplantation: This procedure allows the patient
to retain his own heart but healing of the airway anastomoses may be jeopardised
as a result of ischaemia secondary to interruption of the coronary to
bronchial collateral circulation. BSLT is performed more frequently world
wide than HLT. The procedure is usually performed via bilateral thoracotomy
incisions or via a thoraco-sternotomy (clamshell) incision. The clamshell
incision provides excellent exposure to the thoracic cavity allowing the
surgeon to deal with pleural adhesions. The lungs are inserted in a sequential
fashion and bi-bronchial anastomoses are fashioned in addition to pulmonary
arterial and venous anastomoses. The procedure can often be undertaken
without cardiopulmonary bypass and thus avoids attendant complications
such as bleeding, complement activation and neurological defects. Double
lung transplantation (DLT) is performed on cardiopulmonary bypass and
a tracheal anastomosis is performed.
Single lung transplantation: SLT is the most commonly performed
pulmonary transplant operation worldwide. This is performed through a
postero-lateral thoracotomy. Anastomoses are made at the level of the
main bronchus, pulmonary artery and pulmonary veins (with a cuff of donor
atrium to recipient left atrium).
Choice of operation: The current indications for HLT, DLT, BSLT
and SLT are listed in Table 2. The primary indication for SLT is restrictive
lung disease e.g. pulmonary fibrosis. The increase in elastic recoil and
vascular resistance of the remaining fibrotic lung in these patients ensures
a progressive shift of alveolar ventilation and lung perfusion from the
native to the transplanted side. Ventilation perfusion mismatching is
therefore uncommon. BSLT may be a better alternative for patients with
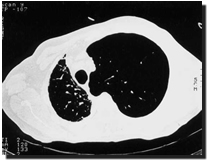
emphysema (Figure 1). Although patients with primary pulmonary hypertension
may be treated successfully with SLT the development of pulmonary oedema
in the transplanted lung in the early postoperative period is associated
with a high mortality. As a consequence of this risk many centres advocate
BSLT or HLT for primary pulmonary hypertension. In general patients who
have pulmonary hypertension require replacement of both lungs if the mean
pulmonary artery pressure exceeds 50mmHg. Pre-existing sepsis e.g. in
cystic fibrosis or bronchiectasis usually precludes SLT because there
is a high risk of the transplanted lung becoming infected by sputum overspill
from the remaining native lung. SLT with pneumonectomy is not considered
appropriate for suppurative lung diseases because of the high risk of
bronchial stump dehiscence and empyema in an immunocompromised host.
Lobar transplantation: Living related lobar transplantation has been
applied successfully to patients with cystic fibrosis. Following bilateral
pneumonectomy the recipient received a bilateral sequential transplant
of a lower lobe from each of two living donors. Intermediate term results
are comparable to cadaveric lung transplantation with respect to survival,
function and incidence of complications.
Question 2.
Bilateral sequential lung transplantation is:
|
|
||
| 1. More frequently performed worldwide than HLT | ||
| 2. Performed usually using cardiopulmonary bypass | ||
| 3. Is indicated for patients with Eisenmenger’s syndrome with a surgically incorrectable cardiac defect | ||
| 4. Is usually performed in patients with pulmonary fibrosis | ||
| 5. Is frequently performed in patients with suppurative lung disease |
DONOR SELECTION
Guidelines to determine organ suitability are listed in Table 3. Good
donor cardiac and respiratory function are essential to optimise success
of cardiac and pulmonary transplantation. The commonest cause of brain
death in donors is trauma with brain injury and cerebral vascular events.
It is appreciated that with increasing demand and scarcity of suitable
donor organs the criteria listed in Table 3 may change. The organ ischaemic
time is the time between placement of the cross clamp on the donor aorta
and reperfusion with the recipient's blood after implantation. The best
results are achieved when this time is less than five hours.
DONOR AND RECIPIENT MATCHING
Matching criteria are based on ABO blood group compatibility, size of
thoracic cage and cytomegalovirus (CMV) antibody status. Potential recipients
are also screened for pre-formed antibodies against a panel of HLA antigens.
However, unlike renal transplantation, formal tissue type matching for
pulmonary transplantation is not practical as it would increase the organ
ischaemic time and furthermore the benefit of HLA matching in pulmonary
transplantation is unclear. Ideally the donor lung should be slightly
smaller than the recipient chest cavity as organs which are too big may
predispose to atelectasis and uneven ventilation due to compression. On
the other hand lungs which are too small may fail to obliterate the plural
space with the potential risk of early plural effusion or empyema formation.
Question 3.
The following are recognised matching criteria for lung transplant recipients:
|
|
|
|
| 1. ABO blood group | ||
| 2. Toxoplasma antibody status | ||
| 3. Cytomegalovirus antibody status | ||
| 4. Size of thoracic cage | ||
| 5. Rhesus factor |
PRETRANSPLANT ASSESSMENT:
Patients are admitted to hospital for a period of about one week which
enables them to get to know the staff, visit the surgical centre and meet
some patients who have already been transplanted. During the assessment
the patients receive a full history and physical examination together
with assessment of psychosocial suitability. Investigations will include
full lung function tests, arterial blood gas analysis on room air, thoracic
CT scan, electrocardiography, transthoracic 2D echocardiography, 24 hour
Holter monitoring, right and left heart catheterisation in selected patients,
routine haematological and biochemical investigations together with serological
investigations for CMV, Epstein-Barr virus, hepatitis A, B and C, toxoplasmosis,
Human Immunodeficiency Virus I & II and herpes simplex. Microbiological
examination of the sputum is undertaken for pathogenic organisms, acid
fast bacilli and fungi.
WAITING LIST
It is essential that patients and their family are fully prepared for
the events which may ensue following acceptance onto the transplant waiting
list. It should be stressed that unfortunately there are more patients
requiring transplantation than suitable donor organs and therefore being
accepted onto the transplant waiting list does not guarantee that a suitable
donor organ will be found for the patient. Indeed up to 40% of patients
die on the transplant waiting list. The patient should be fully advised
of the risks of transplantation and what to expect in the intensive care
unit and during the postoperative period. They should understand that
they will need to take lifelong daily immunosuppressive therapy and will
require careful postoperative surveillance. It is also important to point
out that obliterative bronchiolitis is a potential long term complication.
Once on the waiting list patients face an uncertain time and transplant
support groups are helpful.
POSTOPERATIVE MANAGEMENT
Routine postoperative immunosuppression comprises Azathioprine (or Mycophenolate
Mofetil) and Cyclosporin A (or Tacrolimus). Intravenous Methylprednisolone
is prescribed to treat acute rejection episodes. Acute allograft rejection
is diagnosed by a combination of clinical and radiological findings together
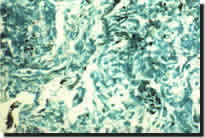
with respiratory function tests and histopathological examination of transbronchial
lung biopsies specimens obtained at fibreoptic bronchoscopy (Figure 2).
It is usually impossible to differentiate allograft rejection from infection
on clinical and radiological grounds alone and therefore bronchoscopy
plays an important role postoperatively. Fibreoptic bronchoscopy is performed
routinely immediately post-transplantation and at the end of the first
postoperative week. Thereafter it is usually only performed if there are
clinical indications as listed in Table 4. At bronchoscopy the anastomosis
is inspected and broncho alveolar lavage specimens are taken for culture
and sensitivity, opportunistic pathogen screen and immunocytochemistry.
Transbronchial lung biopsy specimens are sent for histopathological examination
and culture (Figure 3). Complications of lung transplantation are listed
in Table 5. It can be appreciated that the specialist nature of the majority
of these problems necessitate management by a transplant team. This is
particularly important for cystic fibrosis patients.
Question 4.
Acute allograft rejection is:
|
|
|
|
| 1. Usually diagnosed clinically | ||
| 2. Usually presents with typical radiographic features | ||
| 3. Can be diagnosed by transbronchial lung biopsy | ||
| 4. Occurs more commonly after BSLT and HLT than SLT | ||
| 5. Is usually treated with intravenous Methylprednisolone |
OBLITERATIVE BRONCHIOLITIS
This is the most serious late complication affecting up to 40% of adult
patients within three years of surgery (Figure 4). The incidence is higher
in children who receive transplantation under the age of 10 years. The
diagnosis is made clinically in patients who develop progressive airflow
obstruction often in the presence of infection. Common presenting features
include reduced exercise capacity, cough and progressive deterioration
in lung function. Chest radiography may be normal or may show hyper inflated
lung fields secondary to air trapping.
Transbronchial lung biopsies (Figure 5) are usually not helpful as the
affected bronchioles are randomly distributed throughout the lung and
are peripheral in location and thus not routinely sampled at biopsy. The
diagnosis may be confirmed by DTPA lung scan or high resolution thoracic
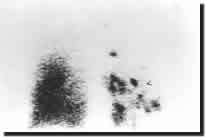
CT scan. DTPA scanning may show patchy uptake and deposition of the radioisotope
in large airways (Figure 6) and a mosaic appearance due to air trapping
may be observed in an expiratory phase CT scan.
The aetiology of obliterative bronchiolitis is unclear but may reflect
a form of chronic allograft rejection. It is also believed that obliterative
bronchiolitis may be a final common pathway to a variety of pulmonary
injuries which may include:
• Recurrent or persistent acute rejection
• Bacterial infection
• Viral infection
• The effects of pulmonary denervation
• Ischaemia.
It is interesting to note that the histological appearances of obliterative
bronchiolitis seen in non-transplant patients with rheumatoid lung or
respiratory syncytial virus infection are similar to those seen in transplant
recipients.
Once the diagnosis of obliterative bronchiolitis is made immunosuppression
is augmented with one or more of the following:
• High dose oral Prednisolone
• Conversion from Cyclosporin A to Tacrolimus or Rapamycin
• Conversion from Azathioprine to Mycophenolate Mofetil
• Total lymphoid irradiation
• IL-2 Receptor antibodies.
Unfortunately the majority of patients will not regain lost-lung function
and will either stabilise at lower levels of lung function or deteriorate
to end stage respiratory failure.
Question 5.
Obliterative bronchiolitis:
|
|
||
| 1. Has a higher incidence in children who receive transplantation under the age of 10 years | ||
| 2. Is usually a clinical diagnosis with progressive airflow obstruction | ||
| 3. Can be confirmed by transbronchial lung biopsies and DTPA and high resolution thoracic CT scans | ||
| 4. Is probably multi-factorial in origin | ||
| 5. Usually responds favourably to augmented immunosuppression |
RESULTS
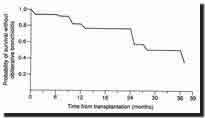
One and two year actuarial survival following both HLT and BSLT is of
the order of 70% and 60% respectively and 80% and 70% respectively following
SLT. Most survivors show a marked improvement in quality of life. Pulmonary
function improves rapidly following surgery and FEV1 and FVC are usually
in excess of 70% predicted following HLT and BSLT by the end of the third
postoperative month. One year actuarial survival of 30% following re-transplantation
for obliterative bronchiolitis has been reported. Such results together
with the current shortage of donor organs worldwide has led many transplant
centres to abandon re-transplantation.
PROGRAMME OF LONG TERM CARE
Following discharge from hospital patients are managed by the transplant
unit in collaboration with the referring centre. Each patient receives
a home micro-spirometer (Figure 7) on discharge and measures FEV1 and
FVC on a daily basis. They are advised to contact the transplant centre
should they develop a:
• Greater than 15% reduction in lung function on home testing on
two consecutive occasions
• Cough
• Pyrexia in excess of 37.5%c
• Reduction in exercise tolerance.
Initially patients attend the transplant centre on a weekly basis but
ultimately the frequency of outpatient appointments becomes less and less
and eventually the majority of patients will attend the transplant centre
for review every six months.
The referring centre is encouraged to play an active role in the management
of the lung transplant recipient and indeed should patients develop problems
the majority will present to their local centre. In such situations early
communication and if necessary prompt referral to the transplant centre
is essential.
If a transplant patient presents unwell to an Accident & Emergency
Department the following approach may be useful:
• Treat any acute medical emergency.
• Take a full history including details of home spirometry record
and perform a physical examination.
• Check FBC, U&E, LFTs, chest x-ray, arterial blood gas analysis
on room air.
• Discuss the clinical presentation and above results with the transplant
centre before deciding on further management.
Question 6.
Postoperatively lung transplant recipients:
|
|
||
| 1. Are usually managed by the referring centre alone | ||
| 2. Receive a home micro-spirometer to measure FEV1 and FVC on a daily basis | ||
3. Have a one year actuarial survival of 50% |
||
| 4. Attain FEV1 and FVC following HLT and BSLT of 70% predicted at the end of the third postoperative month | ||
| 5. Require lifelong immunosuppression |
THE FUTURE
The major challenges facing lung transplant programmes are:
• Shortage of suitable donor organs
• The development of obliterative bronchiolitis
• The timing of surgery.
It is hoped that the development of new immunosuppressive agents together
with improved diagnosis and treatment of rejection and pulmonary infection
will reduce the incidence of obliterative bronchiolitis. Major immunological
issues and concerns over possible transmission of infection have to be
overcome before the use of animal organs (xenografting) can be successfully
applied to human lung transplantation.
LEGENDS
| Click on the image thumbnails for a full size version. | |
|
Figure 1. |
|
|
Figure 2. |
|
|
Figure 3. |
|
|
Figure 4. |
|
|
Figure 5. |
|
|
Figure 6. |
|
|
Figure 7. |
|
Table 1.
Contraindications to lung transplantation
Strong Contra-indications
1. Non-compliance with treatment.
2. Infection with Human Immunodeficiency Virus I & II..
3. Hepatitis B surface antigen and Hepatitis C seropositivity.
4. Active aspergillus or mycobacterial infection.
5. Malignant disease within five years.
6. Bacterial species in sputum with no in-vitro antibiotic sensitivities.
7. Gross malnutrition.
8. Other end organ failure.
9. Prednisolone therapy > 10mg/d.
10. Age > 60 years. *
11. Significant osteoporosis
Risk Factors
1. Chemical pleurodesis.
2. Pre-operative ventilation.
3. Previous thoracic surgery (pleurectomy, abrasion pleurodesis).
4. Severe liver disease necessitating combined heart-lung and liver transplantation.
* Centres may have different policies and some have reported upper age
limits of
65, 60 and 55 years respectively for SLT, BSLT and HLT.
Table 2. (The numbers within the table cells in tables 2a. and 2b.
refer to the annotation below the tables)
Current indications for heart-lung, double-lung and
single-lung transplantation
Table 2a. Parenchymal lung disease
Primary indication |
Alternative options | |
| Cystic Fibrosis | BSLT | DLT, HLT |
| Bronchiectasis | BSLT | DLT, HLT |
| Emphysema | BSLT | SLT, DLT |
| Sarcoidosis | SLT | DLT, BSLT |
| Cryptogenic Fibrosing Alveolitis | SLT | |
| Occupational Lung Disease | SLT | |
| Obliterative Bronchiolitis | SLT | |
| Lymphangioleiomyomatosis | SLT | DLT, BSLT |
| Eosinophilic Granuloma | DLT, BSLT | |
| Adult Respiratory Distress Syndrome | SLT_ 1 |
Table 2b. Pulmonary vascular disease
| Primary indication | Alternative options | |
| Eisenmenger’s syndrome | HLT | DLT or SLT with repair of defect |
| Primary pulmonary hypertension | DLT, BSLT,HLT | SLT_2 |
| Complex pulmonary atresia | HLT | |
| Thromboembolic pulmonary hypertension | DLT | HLT, SLT_2 |
| Pulmonary veno-occlusive disease | HLT_ | 3 |
HLT -----heart-lung transplantation
DLT ---- double-lung transplantation
SLT ----- single-lung transplantation
BSLT --- bilateral sequential lung transplantation.
1. Results of transplantation in the acute phase
of the illness are poor.
2. Reperfusion pulmonary oedema in the early post-operative period is
a serious risk.
3. Experience limited.
Table 3.
Donor selection
1. Brain stem death
2. No significant cardiac or pulmonary injury.
3. Clear lung fields on chest radiograph.
4. Age < 50 years.
5. Non-smoker.
6. Normal gas exchange (PaO2 > 15 kPa with an FiO2 of 35% and PaO2
> 40kPa on FiO2 of 100% and Peep of 5cm H2O).
7. No systemic or endobronchial infection or pneumonia.
8. No past history of pulmonary, cardiac or malignant disease.
9. Normal ECG.
10. No aspiration
Table 4.
Indications for bronchoscopy in lung transplant recipients
1. Reduction in lung function.
2. Reduction in exercise capacity
3. Unexplained cough.
4. Abnormality on chest radiograph
5. Unexplained pyrexia.
Table 5.
Complications of lung transplantation
General
1. Infection.
2. Acute rejection.
3. Airway complications.
4. Bleeding.
5. Multiple organ failure.
6. Complications of immunosuppression e.g. renal failure, bone marrow
suppression.
7. Obliterative bronchiolitis.
8. Lympho-proliferative disorders.
Specific Cystic Fibrosis Related Problems
1. Malnutrition.
2. Liver disease.
3. Salt loss.
4. Diabetes Mellitus.
5. Persisting infection in upper respiratory tract.
6. Malabsorption of cyclosporin A.
7. Meconium ileus equivalent.
REFERENCES
1. Lung Transplantation and Thoracic Surgery
Madden BP
Respiratory Medicine Specialist Handbook
Editors Dilworth P, Baldwin D
Harwood Academic Publication 2001; Pg. 545-565
2. Lung Transplantation
Madden BP
Cystic Fibrosis 2nd Edition
Editors Hodson ME, Geddes DM
Arnold, London 2000; Pg. 361-374
3. Living Related Lung Lobar Transplantation
Madden BP
Surgery 2001; 19:6 : i-ii
4. Intermediate Term Results of Heart-Lung Transplantation for Cystic
Fibrosis
Madden BP, Hodson ME, Tsang V et al.
Lancet 1992; 339:1583-7
5. Medium Term Results of Heart and Lung Transplantation
Madden BP, Radley-Smith R, Hodson M et al.
J Heart Lung Transplant 1992; 11:S241-3
6. Living Donor Lobar Lung Transplantation Experience: Intermediate Results
Starnes VA, Barr M, Cohen R et al.
J Thorac Cardiovasc Surg 1996;112:1284-1291
7. Transbronchial Biopsy in Heart and Lung Transplantation: Clinicopathologic
Correlations
Pomerance A, Madden BP, Burke M, Yacoub M,
J Heart Lung Transplant 1995:14:761-73
8. The Medical Management of Patients with Cystic Fibrosis Following Heart
and Lung Transplantation
Madden BP, Kamalvand K, Chan CM et al.
Eur Respir J 1993:6:965-70
9. Immunology Medicated Disease of the Airways After Pulmonary Transplantation
Griffith BP, Paradis IL, Zeevi et al.
Ann Surg 1988;208:371-9
10. Quality of Well-Being Predicts Survival in Transplantation Candidates
Squier HC, Ries AL, Kaplan RM et al.
Am J Respir Crit Care Med 1995;152:2032-36
11. Indications, Unilateral, Bilateral, Heart-lung and Lobar Transplant
Procedures
Patterson GA
Clin Chest Med 1997; 18 (2): 225-230
12. Influence of HLA Matching on Thoracic Transplant Outcomes. An analysis
from the UNOS/ISHLT Thoracic Registry
Hosenpud JD, Edwards EB, Lin MH, Daily OP
Circulation 1996; 94 (2): 170-174
13. International Guidelines for Selection of Lung Transplant Candidates
Maurer J, Frost A, Estenne M et al
J Heart Lung Transplant 1999; 17: 703-709
14. Early and Longterm Functional Outcomes in Unilateral, Bilateral and
Living-Related Transplant Recipients
Williams TJ, Snell GI
Clin Chest Med 1997; 18 (2): 245-257